 |
SAPIENZA |
Domanda di finanziamento per PROGETTI di RICERCA |
|
 |
SAPIENZA |
Domanda di finanziamento per PROGETTI di RICERCA |
|
|
TRITAPEPE (cognome) |
Luigi (nome) |
|
Professore Associato confermato (qualifica) |
06/03/1959 (data di nascita) |
|
Chirurgia Generale e Specialistica (Dipartimento) |
|
|
Viale del Policlinico, 155 00161 ROMA (indirizzo) |
Macroarea (delibera del S.A del 15.2.2011) |
|
06-49972692 (telefono) |
06-49972595 (fax) |
|
luigi.tritapepe@uniroma1.it (e-mail) |
|
|
LS - Scienze della vita |
(*) I responsabili di questa classe dimensionale (Progetti di Ricerca Medi) possono chiedere l'attribuzione motivata di un assegno di ricerca (dell'importo di euro 23.450) che si aggiunge al finanziamento attribuito.
Il numero totale degli assegni di ricerca disponibili complessivamente per progetti Universitari Medi e Grandi è di 60.
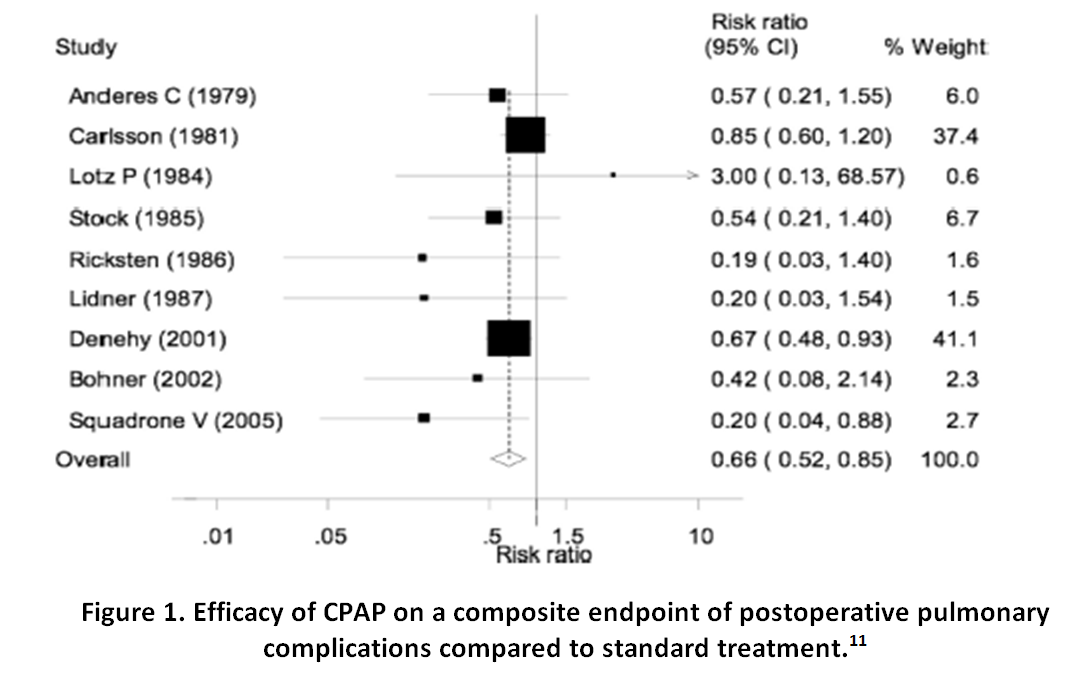
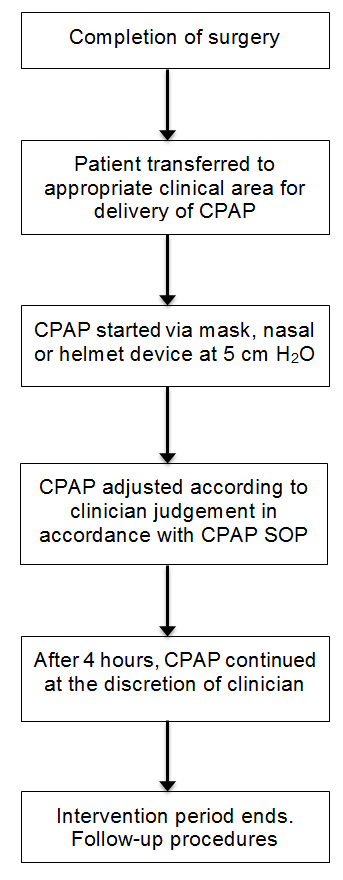
| 1. | CONTINUOUS POSITIVE AIRWAY PRESSURE |
| 2. | RESPIRATORY FAILURE |
| 3. | RESPIRATORY COMPLICATIONS |
| 4. | MAJOR SURGERY |
| 2.2 Ambito della ricerca / Research ambit |
|---|
|
Dipartimento |
| nº | Cognome | Nome | Qualifica | Facoltà | Dipartimento | Macro settore ERC |
|---|---|---|---|---|---|---|
| 1. | MORELLI | Andrea | Professore Associato (L. 240/10) | DIP. Scienze cardiovascolari, respiratorie, nefrologiche, anestesiologiche e geriatriche | LS | |
| 2. | RANIERI | Vito Marco | Professore Ordinario (L. 240/10) | DIP. Scienze cardiovascolari, respiratorie, nefrologiche, anestesiologiche e geriatriche | LS |
| nº | Cognome | Nome | Qualifica | Dipartimento | Macro settore ERC |
|---|
Non inserire in questo punto PERSONALE DOCENTE e RICERCATORE strutturato, pena l’esclusione della domanda per VIZIO DI FORMA.
| nº | Cognome | Nome | Qualifica | Dipartimento | Note |
|---|
| nº | Cognome | Nome | Qualifica | Universita'/Istituzione | Dipartimento | Note |
|---|
| H-INDEX | Database |
|---|---|
(Le pubblicazioni dall'anno 2014 non riportano l'impact factor)
| nº | Descrizione | Impact Factor |
|---|---|---|
| 1. | G.Illuminati, F.Schneider, C.Greco, E.Mangieri, M.Schiariti, G.Tanzilli, F.Barillà, V.Paravati, G.Pizzardi, F.Calio', F.Miraldi, F.Macrina, M.Totaro, E.Greco, G.Mazzesi, L.Tritapepe, M.Toscano, F.Vietri, N.Meyer, J-B.Ricco (2015). Long-term results of a randomized controlled trial analyzing the role of systematic pre-operative coronary angiography before elective carotid endarterectomy in patients with asymptomatic coronary artery disease.. EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY, vol. 49, p. 366-374, ISSN: 1078-5884, doi: dx.doi.org/10.1016/j.ejvs.2014.12.030 | |
| 2. | Del Porto F, Proietta M, di Gioia C, Cifani N, Dito R, Fantozzi C, Ferri L, Fabriani L, Rossi M, Tritapepe L, Taurino M (2015). FGF-23 levels in patients with critical carotid artery stenosis.. INTERNAL AND EMERGENCY MEDICINE, ISSN: 1970-9366 | |
| 3. | M. Falcone, A. Russo, M. Mancone, G. Carriero, G. Mazzesi, F. Miraldi, M. Pennacchi, F. Pugliese, L. Tritapepe, V. Vullo, F. Fedele, G. Sardella, M. Venditti. (2014). Early, intermediate and late infectious complications after transcatheter or surgical aortic-valve replacement: a prospective cohort study.. CLINICAL MICROBIOLOGY AND INFECTION, vol. 20, p. 758-763, ISSN: 1198-743X | |
| 4. | Morabito S, Pistolesi V, Tritapepe L, Fiaccadori E. (2014). Regional Citrate Anticoagulation for RRTs in Critically Ill Patients with AKI.. CLINICAL JOURNAL OF THE AMERICAN SOCIETY OF NEPHROLOGY, p. 2173-2188, ISSN: 1555-9041 | |
| 5. | M. Falcone, A. Russo, M. Mancone, G. Carriero, G. Mazzesi, F. Miraldi, M. Pennacchi, F. Pugliese, L. Tritapepe, V. Vullo, F. Fedele, G. Sardella, M. Venditti (2014). Early, intermediate and late infectious complications after transcatheter or surgical aortic-valve replacement: a prospective cohort study. CLINICAL MICROBIOLOGY AND INFECTION, vol. 8, p. 758-763, ISSN: 1198-743X, doi: 10.111/1469-0691.12470 | |
| 6. | Vincenzo De Santis, Giacomo Frati, Ernesto Greco, Luigi Tritapepe (2014). Ivabradine: a preliminary observation for a new terapeutic role in patients with multiple organ dysfunction syndrome. CLINICAL RESEARCH IN CARDIOLOGY, vol. 103, p. 831-834, ISSN: 1861-0684, doi: 10.1007/s00392-014-0722-2 | |
| 7. | Flavia Del Porto, Cira Rosaria Tiziana Di Gioia, Luigi Tritapepe, Livia Ferri, Martina Leopizzi, Italo Nofroni, Vincenzo De Santis, Carlo Della Rocca, Anna Paola Mitterhofer, Guglielmo Bruno, Maurizio Taurino, Maria Proietta (2014). The Multitasking Role of Macrophages in Stanford Type A Acute Aortic Dissection. CARDIOLOGY, vol. 127, p. 123-129, ISSN: 0008-6312, doi: 10.1159/000355253 | |
| 8. | Maria Proietta, Luigi Tritapepe, Noemi Cifani, Livia Ferri, Maurizio Taurino, Flavia Del Porto (2014). MMP-12 as a new marker of Stanford-A acute aortic dissection. ANNALS OF MEDICINE, vol. 46, p. 44-48, ISSN: 0785-3890, doi: 10.3109/07853890.2013.876728 | |
| 9. | Andrea Morelli, Christian Ertmer, Martin Westphal, Sebastian Rehberg, Tim Kampmeier, Sandra Ligges, Alessandra Orecchioni, Annalia D'Egidio, Fiorella D'Ippoliti, Cristina Raffone, Mario Venditti, Fabio Guarracino, Massimo Girardis, Luigi Tritapepe, Paolo Pietropaoli, Alexander Mebazaa, Mervyn Singer (2013). Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial.. JAMA, vol. 310, p. 1683-1691, ISSN: 0098-7484, doi: 10.1001/jama.2013.278477 | |
| 10. |
Santo Morabito, Valentina Pistolesi, Luigi Tritapepe, Laura Zeppilli, Francesca Polistena, Emanuela Strampelli, Alessandro Pierucci (2012). Regional citrate anticoagulation in cardiac surgery patients at high risk of bleeding: a continuous veno-venous hemofiltration protocol with a low concentration citrate solution. CRITICAL CARE, vol. 16, ISSN: 1466-609X, doi: 10.1186/cc11403 |
4,718 |
| 11. | Vincenzo De Santis, Domenico Vitale, Anna Santoro, Aurora Magliocca, Andrea Giuseppe Porto, Cecilia Nencini, Luigi Tritapepe (2012). Ivabradine: potential clinical applications in critically ill patients. CLINICAL RESEARCH IN CARDIOLOGY, ISSN: 1861-0684, doi: 10.1007/s00392-012-0516-3 | 3,667 |
| 12. | F. Guarracino, Luigi Tritapepe (2011). Is preoperative levosimendan indicated to treat normal left ventricular function and left ventricle outflow obstruction?. BRITISH JOURNAL OF ANAESTHESIA, vol. 107, p. 276-277, ISSN: 0007-0912, doi: 10.1093/bja/aer212 | 4,243 |
| 13. | DE SANTIS V, VITALE D, L. TRITAPEPE (2010). Levosimendan and cardiac surgery.. JOURNAL OF CARDIOTHORACIC AND VASCULAR ANESTHESIA, vol. 24, p. 210, ISSN: 1053-0770, doi: 10.1053/j.jvca.2009.01.008 | 1,596 |
| 14. |
ILLUMINATI G, RICCO JB, GRECO C, MANGIERI E, CALIO' F, CECCANEI G, PACILÈ MA, SCHIARITI M, TANZILLI G, F. BARILLA', PARAVATI V, MAZZESI G, MIRALDI F, TRITAPEPE L (2010). Systematic preoperative coronary angiography and stenting improves postoperative results of carotid endarterectomy in patients with asyntomatic coronary artery disease: a randomised controlled trial. . EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY, vol. Feb;39(2), p. 139-145, ISSN: 1078-5884, doi: 10.1016/j.ejvs.2009.11.015 |
2,872 |
| 15. | DE SANTIS V, NENCINI C, L. TRITAPEPE (2010). Comparison of dopamine and norepinephrine in shock.. NEW ENGLAND JOURNAL OF MEDICINE, vol. 362, p. 2330-2331, ISSN: 0028-4793, doi: 10.1056/NEJMc1003900 | 53,486 |
| nº | Pubblicazione | Docente |
|---|---|---|
| 1. | Andrea Morelli, Abele Donati, Christian Ertmer, Sebastian Rehberg, Tim Kampmeier, Alessandra Orecchioni, Alessandro Di Russo, Annalia D'Egidio, Giovanni Landoni, Maria Lombrano, Laura Botticelli, Agnese Valentini, Alberto Zangrillo, Paolo Pietropaoli, Martin Westphal (2011). Effects of vasopressinergic receptor agonists on sublingual microcirculation in norepinephrine-dependent septic shock. CRITICAL CARE, vol. 15, ISSN: 1466-609X, doi: 10.1186/cc10453 | MORELLI Andrea |
| 2. | Andrea Morelli, Abele Donati, Christian Ertmer, Sebastian Rehberg, Matthias Lange, Alessandra Orecchioni, Valeria Cecchini, Giovanni Landoni, Paolo Pelaia, Paolo Pietropaoli, Hugo Van Aken, Jean-Louis Teboul, Can Ince, Martin Westphal (2010). Levosimendan for resuscitating the microcirculation in patients with septic shock: a randomized controlled study. CRITICAL CARE, vol. 14, ISSN: 1466-609X, doi: 10.1186/cc9387 | MORELLI Andrea |
| 3. | Giovanni Landoni, Giuseppe Biondi Zoccai, Massimiliano Greco, Teresa Greco, Elena Bignami, Andrea Morelli, Fabio Guarracino, Alberto Zangrillo (2012). Effects of levosimendan on mortality and hospitalization. A meta-analysis of randomized controlled studies. CRITICAL CARE MEDICINE, vol. 40, p. 634-646, ISSN: 0090-3493, doi: 10.1097/ccm.0b013e318232962a | MORELLI Andrea |
| 4. | Morelli A, Donati A, Ertmer C, Rehberg S, Kampmeier T, Orecchioni A, Di Russo A, D'Egidio A, Landoni G, Lombrano MR, Botticelli L, Valentini A, Zangrillo A, Pietropaoli P, Westphal M (2011). Effects of vasopressinergic receptor agonists on sublingual microcirculation in norepinephrine-dependent septic shock. CRITICAL CARE, vol. 15(5), ISSN: 1364-8535, doi: 10.1186/cc10453 | MORELLI Andrea |
| 5. | Papp Z, Édes I, Fruhwald S, De Hert SG, Salmenperä M, Leppikangas H, Mebazaa A, Landoni G, Grossini E, Caimmi P, Morelli A, Guarracino F, Schwinger RH, Meyer S, Algotsson L, Wikström BG, Jörgensen K, Filippatos G, Parissis JT, González MJ, Parkhomenko A, Yilmaz MB, Kivikko M, Pollesello P, Follath F (2012). Levosimendan: Molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. INTERNATIONAL JOURNAL OF CARDIOLOGY, vol. 159(2), p. 82-87, ISSN: 0167-5273, doi: 10.1016/j.ijcard.2011.07.022 | MORELLI Andrea |
| 6. | Morelli A, Donati A, Ertmer C, Rehberg S, Orecchioni A, Di Russo A, Pelaia P, Pietropaoli P, Westphal M (2011). Short-term effects of terlipressin bolus infusion on sublingual microcirculatory blood flow during septic shock. INTENSIVE CARE MEDICINE, vol. 37(6), p. 963-969, ISSN: 0342-4642, doi: 10.1007/s00134-011-2148-x | MORELLI Andrea |
| 7. | Andrea Morelli, Christian Ertmer, Martin Westphal, Sebastian Rehberg, Tim Kampmeier, Sandra Ligges, Alessandra Orecchioni, Annalia D'Egidio, Fiorella D'Ippoliti, Cristina Raffone, Mario Venditti, Fabio Guarracino, Massimo Girardis, Luigi Tritapepe, Paolo Pietropaoli, Alexander Mebazaa, Mervyn Singer (2013). Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial.. JAMA, vol. 310, p. 1683-1691, ISSN: 0098-7484, doi: 10.1001/jama.2013.278477 | MORELLI Andrea |
| 8. | Morelli A, Ertmer C, Singer M (2014). Short-acting β-blocker administration in patients with septic shock--reply.. JAMA, vol. 311, ISSN: 0098-7484, doi: 10.1001/jama.2014.327 | MORELLI Andrea |
| 9. | Guarracino F, Ferro B, Morelli A, Bertini P, Baldassarri R, Pinsky MR (2014). Ventriculo-arterial decoupling in human septic shock.. CRITICAL CARE, vol. 18, ISSN: 1364-8535, doi: 10.1186/cc13842 | MORELLI Andrea |
| 10. | Andrea Morelli, Abele Donati, Christian Ertmer, Sebastian Rehberg, Tim Kampmeier, Alessandra Orecchioni, Annalia D'Egidio, Valeria Cecchini, Giovanni Landoni, Paolo Pietropaoli, Martin Westphal, Mario Venditti, Alexandre Mebazaa, Mervyn Singer (2013). Microvascular effects of heart rate control with esmolol in patients with septic shock: a pilot study.. CRITICAL CARE MEDICINE, vol. 41, p. 2162-2168, ISSN: 0090-3493, doi: 10.1097/ccm.0b013e31828a678d | MORELLI Andrea |
| 11. | Slutsky AS, Ranieri VM. (2013). Ventilator-induced lung injury. NEW ENGLAND JOURNAL OF MEDICINE, vol. 22, p. 2126-2136, ISSN: 0028-4793, doi: 10.1056/NEJMra1208707 | RANIERI Vito Marco |
| 12. | Rautanen A, Mills TC, Gordon AC, Hutton P, Steffens M, Nuamah R, Chiche JD, Parks T, Chapman SJ, Davenport EE, Elliott KS, Bion J, Lichtner P, Meitinger T, Wienker TF, Caulfield MJ, Mein C, Bloos F, Bobek I, Cotogni P, Sramek V, Sarapuu S, Kobilay M, Ranieri VM, Rello J, Sirgo G, Weiss YG, Russwurm S, Schneider EM, Reinhart K, Holloway PA, Knight JC, Garrard CS, Russell JA, Walley KR, Stüber F, Hill AV, Hinds CJ, ESICM/ECCRN GenOSept Investigators (2015). Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study.. THE LANCET RESPIRATORY MEDICINE, vol. 3, p. 53-60, ISSN: 2213-2600, doi: 10.1016/S2213-2600(14)70290-5 | RANIERI Vito Marco |
| 13. | Slutsky AS, Ranieri VM (2014). Ventilator-induced lung injury.. NEW ENGLAND JOURNAL OF MEDICINE, vol. 370, p. 980, ISSN: 0028-4793, doi: 10.1056/NEJMc1400293 | RANIERI Vito Marco |
| 14. | Martin EL, Ranieri VM. (2011). Phosphorylation mechanisms in intensive care medicine. INTENSIVE CARE MEDICINE, vol. 37 (1), p. 7-18, ISSN: 0342-4642 | RANIERI Vito Marco |
| 15. | Ranieri VM, Rubenfeld GD, Thompson BT (2013). Defining ARDS: do we need a mandatory waiting period?. INTENSIVE CARE MEDICINE, vol. 39, p. 775-778, ISSN: 0342-4642, doi: 10.1007/s00134-013-2834-y | RANIERI Vito Marco |
| 16. | V. Marco Ranieri, B. Taylor Thompson, Philip S. Barie, Jean-François Dhainaut, Ivor S. Douglas, Simon Finfer, Bengt Gårdlund, John C. Marshall, Andrew Rhodes, Antonio Artigas, Didier Payen, Jyrki Tenhunen, Hussein R. Al-Khalidi, Vivian Thompson, Jonathan Janes, William L. Macias, Burkhard Vangerow, Mark D. Williams (2012). Drotrecogin Alfa (Activated) in Adults with Septic Shock. NEW ENGLAND JOURNAL OF MEDICINE, vol. 366, p. 2055-2064, ISSN: 0028-4793, doi: 10.1056/NEJMoa1202290 | RANIERI Vito Marco |
| 17. | Rupert M Pearse, Rui P Moreno, Peter Bauer, Paolo Pelosi, Philipp Metnitz, Claudia Spies, Benoit Vallet, Jean-Louis Vincent, Andreas Hoeft, Andrew Rhodes, Ranieri VM (2012). Mortality after surgery in Europe: a 7 day cohort study. THE LANCET, vol. 380, p. 1059-1065, ISSN: 0140-6736, doi: 10.1016/S0140-6736(12)61148-9 | RANIERI Vito Marco |
| 18. | De Rosa FG, Corcione S, Pagani N, Stella ML, Urbino R, Di Perri G, Ranieri VM (2013). High rate of respiratory MDR gram-negative bacteria in H1N1-ARDS treated with ECMO.. INTENSIVE CARE MEDICINE, vol. 39, p. 1880-1881, ISSN: 0342-4642, doi: 10.1007/s00134-013-3012-y | RANIERI Vito Marco |
| 19. | Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D, Grasso S, Chiumello D, Guerín C, Patroniti N, Ranieri VM, Gattinoni L, Nava S, Terragni PP, Pesenti A, Tobin M, Mancebo J, Brochard L (2014). The Application of Esophageal Pressure Measurement in Patients with Respiratory Failure.. AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE, vol. 189, p. 520-531, ISSN: 1073-449X, doi: 10.1164/rccm.201312-2193CI | RANIERI Vito Marco |
| 20. | M. Boffini, D. Ricci, R. Bonato, V. Fanelli, M. Attisani, M. Ribezzo, P. Solidoro, L. Del Sorbo, V. M. Ranieri, M. Rinaldi (2014). Incidence and severity of primary graft dysfunction after lung transplantation using rejected grafts reconditioned with ex vivo lung perfusion.. EUROPEAN JOURNAL OF CARDIO-THORACIC SURGERY, vol. 46, p. 789-793, ISSN: 1010-7940, doi: 10.1093/ejcts/ezu239 | RANIERI Vito Marco |
| SPESA IN EURO / COST | Descrizione / Description | |
|---|---|---|
| Materiale inventariabile (comprese le pubblicazioni da acquisire)/Durable Equipments (publications included) | 0,00 | None related to the project |
| Materiale di consumo e funzionamento / Materials & Consumables | 1.000,00 | Materials & Consumables |
| Spese per calcolo ed elaborazione dati / Computing & Data Processing Cost | 1.000,00 | Computing & Data Processing Cost |
| Personale a contratto per supporto alla ricerca o visitatore / Labour | 0,00 | None related to the project |
| Missioni e partecipazioni a convegni / Travels & participation to conferences & workshops | 1.000,00 | Travels & participation to conferences |
| Organizzazione convegni / Subsistence | 0,00 | None related to the project |
| Spese per stampa pubblicazioni / Publications cost | 0,00 | None related to the project |
| Altro (voce da utilizzare solo in caso di spese non riconducibili alle voci sopraindicate – es. over head) / Other costs | 2.000,00 | Other costs related to the project |
| TOTALE | 5.000,00 |
| Fondo assegnato | Fondo non ancora utilizzato | |
|---|---|---|
| Progetto Universitario 2013 | ||
| Progetto Universitario 2012 |
I consuntivi 2013 dei fondi di Università devono essere compilati a parte tramite lo specifico modulo.
| In caso di assegnazione del finanziamento il sottoscritto accetta che titolo della ricerca, abstract e finanziamento assegnato vengano resi pubblici | SI |
|---|---|
| Indirizzo e-mail del Direttore di Dipartimento | carlo.gaudio@uniroma1.it |
| Indirizzo e-mail del Segratario amministrativo di Dipartimento | tommaso.prograno@uniroma1.it |
|
Data 08/05/2015 13:42 |